Two years safeguarding the Pfizer-BioNTech COVID-19 vaccine
In the first two years, more than five billion doses of the Pfizer-BioNTech vaccine had been administered around the world, with Controlant’s solution helping to achieve a 99.99% successful delivery rate.
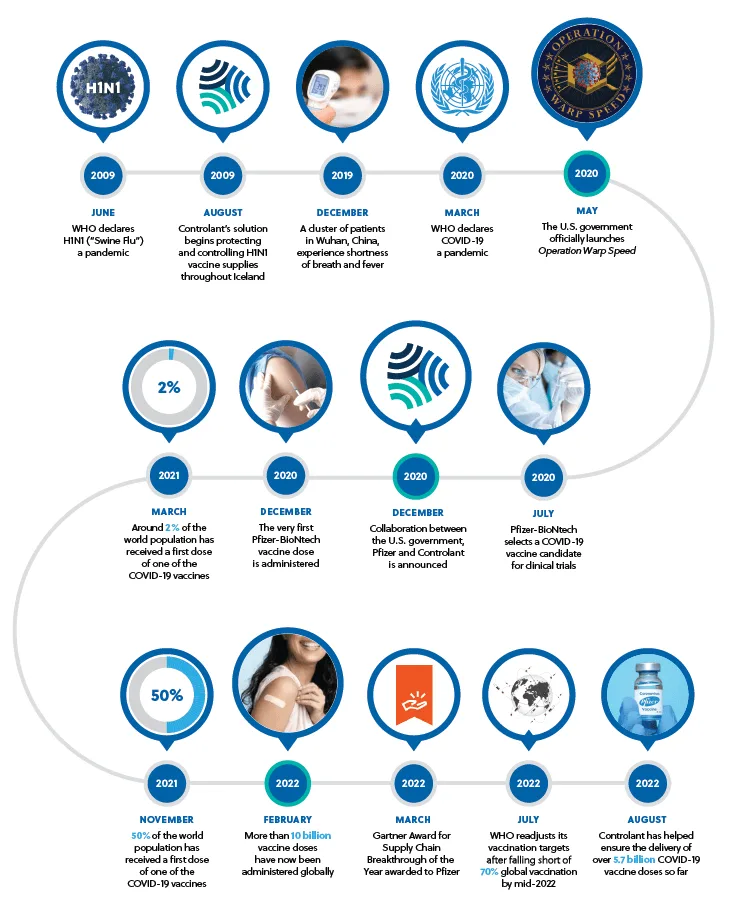
Key milestones along the way say a lot about the collaboration between Pfizer and Controlant, and about the extraordinary achievements of these past two years, as we continue to meet ongoing supply chain challenges.
The vaccine race
20 April 2020 - As several pharmaceutical companies have initiated R&D processes to find a COVID-19 vaccine that can prevent further spread of the novel coronavirus, it is predicted that at the rapid pace at which researchers are currently working, a treatment could be available within 18 months and possibly sooner.
Read more
The first vaccines would be given emergency approval and administered with 8 months, cutting 10 months off an already ambitious target. Today, more than 12 billion COVID-19 vaccine doses from numerous providers have been administered globally. The World Health Organization had set a target of 70% of the world population vaccinated by mid-2022. The reality is closer to 61.5% but almost 70% have at least received their first dose, with a current average of roughly 10 million doses administered globally each day.
Pfizer and Operation Warp Speed
The US government officially launched Operation Warp Speed in May 2020 to set targets for and coordinate the production and delivery of COVID-19 vaccines, which led to the advance-purchase order of the Pfizer–BioNTech vaccine two months later. Pfizer had quickly established itself as a global leader in COVID-19 vaccine development and distribution. Months before clinical trials on its vaccine began, predicting that global demand for the vaccine would be huge, Pfizer ramped up its manufacturing capability in Europe and the US.
New processes were designed, new labs and production lines were built, hundreds of deep freezers were bought. Pfizer remains at the forefront, having created an anti-viral medication to treat the virus now too.
As the pharma world revved up vaccine development back in 2020, the industry’s focus on the advantages of real-time cold chain monitoring quickly sharpened. It also became clear that tight collaboration between manufacturers, distributors, LSPs, governments, and healthcare facilities would be essential in preparing to distribute the vaccine.
15 December 2020 - “Controlant’s 24/7 visibility and monitoring solutions fundamentally change the temperature-controlled supply chain from a reactive operational function to a proactive function, providing stakeholders across a product’s supply chain with an array of solutions to help prevent supply chain-related product loss, tampering, and spoilage, while increasing responsiveness and resilience.”
The internal and external control towers would need to be able to look at a screen at any given moment and know exactly where every shipment is and how cold it is. Operation Warp Speed stakeholders recognized that Controlant’s solutions and service teams could play a critical role in ensuring vaccine quality, improving traceability, and preventing waste through IoT-enabled real-time monitoring, with vital data made accessible to all key players.
18 December 2020 – “Controlant, a leader in real-time supply chain monitoring and visibility for heavily regulated industries […] today announced the company is working with the U.S. Department of Health and Human Services (HHS) and other Operation Warp Speed agency partners to help ensure the availability of COVID-19 vaccines to the American public.”
Not our first rodeo
To meet this challenge, Controlant would need to accelerate its growth, but the company had begun scaling up its operations long before the first reports of a worrying new virus emerged in Wuhan in 2019. This wasn’t our first pandemic response. It wasn’t even the first time we’d implemented a 24/7 vaccine monitoring solution. During the H1N1 (Swine Flu) pandemic in 2009, third-party logistics providers, distribution centers, pharmacies, and health clinics in Iceland used Controlant’s solution to protect and control the transportation and storage of vaccine supplies.
1 March 2018 – “Concerned that the storage coolers located throughout Iceland were not reliable enough to store the vaccine supplies, the Directorate of Health sought out a new solution that could be promptly implemented. Within two weeks, Controlant’s real-time temperature and humidity monitoring solutions were installed […] to monitor vaccine conditions for supplies that needed to be kept between 2-8°C. This ensured product quality, integrity, and safety for end patients.”
Read more
So as an even more serious pandemic emerged in 2020, Controlant’s strategy was grounded in years of experience. Even so, growing fast enough to support Pfizer was an impressive feat.
10 November 2021 – “Under normal circumstances, it can take years to set up a distribution model for accommodating new pharmaceuticals. Controlant accomplished the task in a matter of months, deploying multiple integrated control towers, sharing data across all stakeholders, and implementing processes for tight collaboration all along the supply chain.”
Read more
Long-term results
After this initial sprint, and with the pandemic showing signs it would linger for longer than initially hoped, Controlant and vaccine producers such as Pfizer settled in for the long haul and started looking more closely at the results of this digital transformation. On top of numerous international awards for our technology – from the 2020 SDCE Green Supply Chain Award to the 2022 GLOMO Award for Best Innovation for COVID-19 Pandemic Response and Recovery and making Fast Company’s top ten Most Innovative Companies– Controlant’s customers continue to report dramatic improvements in successful delivery rate, resulting in less waste and greater efficiency, not to mention the health and monetary benefits.
27 July 2021 – “Companies that have started down the path of automation triggered by specific predetermined rules are seeing tremendous outcomes. Automation can cover business, logistics, and quality release processes, for example. It includes automation of communications and data sharing among parties, providing both scalability and sustainability.”
Read more
The pandemic and the resulting collaboration with Pfizer clearly sped up Controlant’s innovation journey and provided us with a great opportunity to demonstrate the benefits of a more intelligent cold chain. As factors including population growth and mobility, increasing consumer demands, and competitive pressures make cold chains increasingly complex, only a centralized solution offers the single source of truth necessary to compete, adapt, and thrive. With Controlant technology, our partners in the pharmaceutical industry gain the power to make strategic decisions that strengthen their supply chain by turning real-time, aggregated data into actionable insights.
Visionary chiefs will lead the effort to digitize the global supply chain and transform their business operations into an agile and efficient business function. […] An automated solution, like Controlant’s, consisting of a centralized platform powered by IoT that can deliver real-time temperature and product movement visibility, provides the data needed for better risk reduction, operational efficiency, and supply chain agility.


