Controlant BioTrack
Our new mobile app gives you real-time visibility of your time-critical biological sample logistics.
The mobile app enables drivers to scan biomedical samples into temperature-controlled transport and monitor the temperature on the go. Drivers receive live alerts for temperature boundary breaches, and samples can easily be added from multiple pickup locations along the route.

Collecting & delivering biomedical samples to labs is time-sensitive and challenging
Ensuring sample viability and safety on-the-go is crucial with only one chance at delivery. Drivers face challenges due to a lack of real-time in-transit notifications during multi-stop routes, an inability to monitor temperatures of different boxes (cold, ambient, dry ice), and the need to accurately track and reconcile thousands of samples.
Key benefits of Controlant BioTrack include:
- Improved sample management
- Fewer damaged samples, saving cost & time
- Increased efficiency in clinical trials
- Increased overview of sample routes, driver utilization & efficiency
Driver-centric mobile app
Drivers can scan samples into temperature-specific boxes (cold, ambient, dry ice), monitor conditions in transit, and receive real-time excursion alerts.
Real-time sample monitoring
Labs can track biosample conditions from pickup to delivery, reducing pre-analytical errors and ensuring that samples arrive viable and test-ready.
Chain of custody & security
BioTrack ensures full traceability with secure handovers via QR code and digital custody logs. It flags tampering, route deviations, and light events.
Improvement in sample integrity benefits many stakeholders
Clinical teams benefit greatly from simplified reconciliation of samples vs. eCRF vs. central lab results. The reduced need for repeated tests positively impacts patients and healthcare workers.
Regulatory compliance at scale
The platform supports GDP/GxP compliance, integrates seamlessly with your systems and workflows, and is validated for use across 200+ airlines.
Fleet & route optimization
Track route timing, driver utilization, and pickup patterns, enabling predictive maintenance and efficient resource allocation.
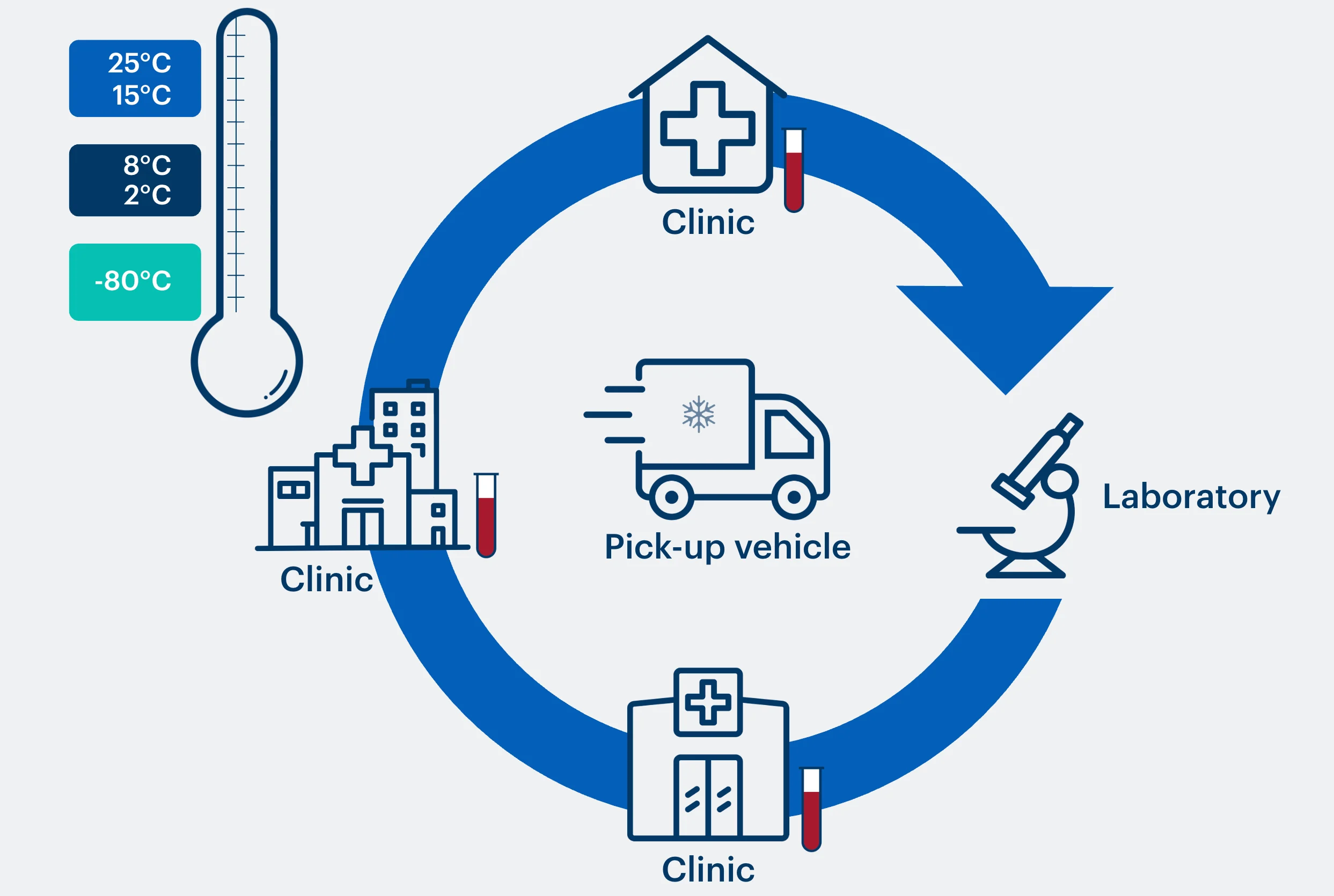
The BioTrack process
Starting a collection route
The driver uses the app to assign a Controlant real-time monitoring device to a storage box. The devices cover all temperature ranges (frozen, chilled, ambient).
Along the collection route
Each pickup is scanned into the box via the app. The driver can react to temperature notifications in real time, and can check samples out of boxes when delivered to lab in a simple one-step process.
Audit-ready documentation
Automated reports and geo-tagged custody logs ensure traceability and regulatory defense, especially important for decentralized trials and multi-site diagnostics.
Easy monitoring & process optimization via the platform
Sample management overview, real-time visibility, and historical route information are available via the platform.
Contact us
Get started with Controlant

